VALIDATION SERVICES
Validation Package
Medical devices manufacturers must guarantee that inspection equipment is suitable for its indented use and capable of producing valid results. This is usually done through Installation Qualification (IQ), Operation Qualification (OQ) and Performance Qualification (PQ) and in the case of automated equipment, it is also required to assure that the software has been validated for its intended use. Software validation and system qualification can be a long and challenging process if medical devices manufacturers don’t have detailed information about the test equipment.
Sensofar Medical validation package is designed to provide the documentation and support needed to validate the inspection equipment and its software in order to have a production-ready system, in compliance with regulatory requirements. The validation services package is comprised of a set of documents for hardware and software validation and a validation service to support manufacturers during qualification activities.
Validation Documentation
Validation documentation contains the information needed to ensure that the software and hardware of the inspection equipment are validated for their intended use.
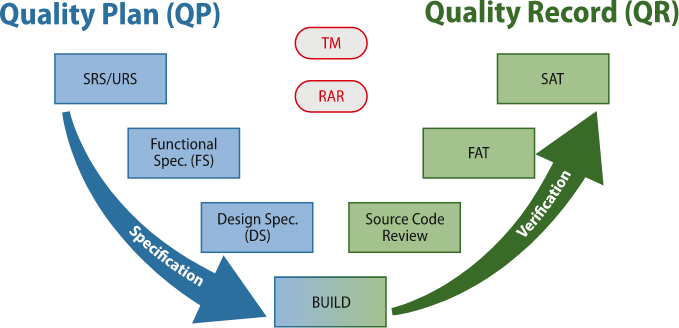
Validation documentation is comprised of the following documents, that can will be adapted to each specific user requirements:
System Requirements Specifications (SRS)
Functional Specifications (FS)
Risk Assessment Report (RAR)
Traceability Matrix (TM)
Validation Report (VR)
Validation Support Service
Sensofar Medical offers support during equipment qualification, helping in the definition of IQ and OQ protocols and being able to execute such protocols. Sensofar Medical can also offer support during the definition of PQ protocol and supervise its execution for as many number of device models as required.