3D label free bio-transfer standards
3D label free bio-transfer standards, full article
Miikka Järvinen,1 Tuomas Vainikka,1 Tapani Viitala,1 Carlos Bermudez,2 Roger Artigas,2 Anton Nolvi,1 Pol Martinez,2 Niklas Sandler,3 Edward Hæggström,1 Ivan Kassamakov1,4
1 Univ. of Helsinki (Finland)
2 Sensofar-Tech, S.L. (Spain)
3 Åbo Akademi Univ. (Finland)
4 Helsinki Institute of Physics (Finland)
Proceedings Volume 10819, Optical Metrology and Inspection for Industrial Applications V; 108190D (2018)
Event: SPIE/COS Photonics Asia, 2018, Beijing, China
Abstract
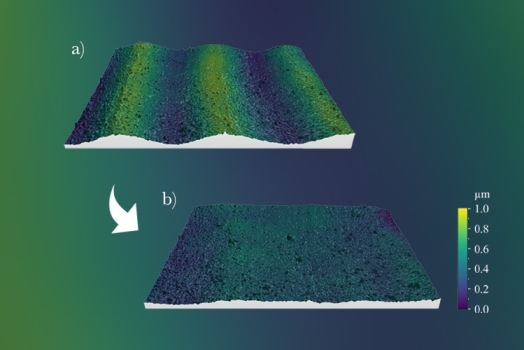
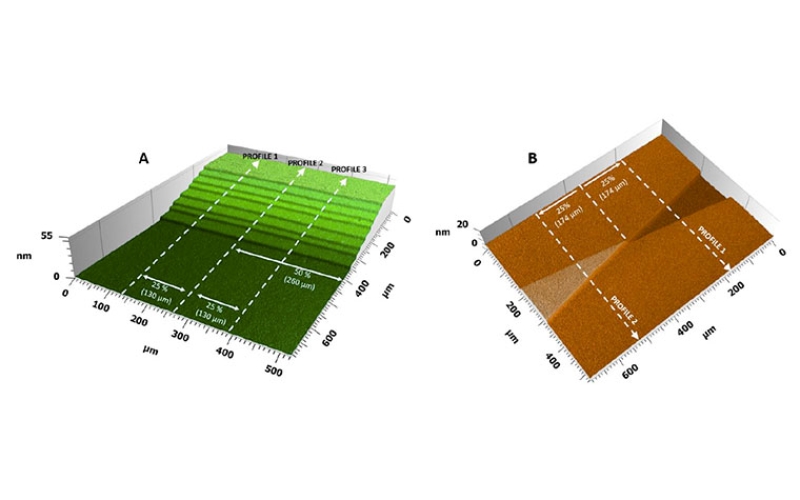
Two kinds of 3D label free Bio-Transfer-Standards (BTS) have been further developed at the University of Helsinki (UH). The first one, NanoRuler, is a staircase BTS featuring eight fatty acid bilayers which allows vertical calibration in the range of 5 to 40 nm. The second one, NanoStar, is a V-shaped BTS featuring two 5 nm tall bilayers that overlap at 10° angle. This standard enables the determination of the Instrument Transfer Function (ITF). A stability test was conducted on the BTSs, during which the standards were stored in laboratory conditions, and were profiled each week. Profiling was done using a custom-built Scanning White Light Interferometer (SWLI). The stability of NanoStar was ± 0.3 nm, and of NanoRuler ± 0.5 nm to ± 2.5 nm. The BTSs maintained their specified properties for at least six months and therefore allow vertical calibration and ITF determination. In addition, changes in surface morphology of one NanoRuler subjected to water immersion are presented. This paper reports intermediate findings during an ongoing stability test that will run for 24 months.